Friday 30 October 2015
Thursday 29 October 2015
Re-purpose Cladribine !
Here's the email I sent Chuka Umunna on 18 Oct:
Dear Mr Umunna
I
am contacting you about the drug re-purposing Bill to ask for your
support to see it become Law. I believe it comes before the house on
November 6. I attach some documents setting out the case for the Bill
and please excuse me if you are already familiar with the arguments.
Breakthroughs
in research have meant that several existing drugs have been found to
be effective in treating conditions other than the ones they were
originally made and patented for. They are referred to as “off patent”
drugs. Repurposing these could have potentially huge benefits for many
people suffering from conditions like multiple sclerosis (MS) and Cancer.
They can be supplied at low cost by Generic Drug companies. The annual
treatment for a patient may be less than £1,000 versus over 20x the cost
for patented alternatives if in fact they exist. The savings would be
of great benefit to patients, the NHS and the taxpayer, and could lead
to treatment for many people whose treatment is denied by NICE on the
grounds of cost.
However,
drugs need to be licensed and approved for their new uses in order to
be made available and there is little commercial incentive for
pharmaceutical companies, who normally sponsor this process, to do so
for off patent treatments. This is largely because prices generally fall
a great deal once the patent has expired. There
is no mechanism in place to enable licensing of drugs for uses other
than their original purpose. Even if a doctor can refer to strong
evidence in support of prescribing an off-patent drug to a patient, they
would likely be put off by the potential personal liability they may
face in doing so.
There
are thus political, legal and commercial barriers in place to
prescribing off patent drugs. The Bill seeks to overcome these barriers
by obliging the Government to act
in the public interest through the process of the Medicines and
Healthcare Regulatory Agency (MHRA) to license and approve off-patent,
repurposed drugs for use on the NHS.
The
Bill is strongly supported by medical charities and specialists in the
NHS, including myself - I am a Reader in Clinical Neurology at Queen Mary University of London &
Consultant Neurologist at Barts Health NHS Trust. As a clinical
academic with an interest in MS, I know that early
effective treatment of people with MS is key for a beneficial long term
outcome. I therefore have a particular interest in the re-purposing of
Cladribine, a drug licensed in the UK for patients with hairy cell
leukaemia. There is evidence from phase III trials that Cladribine is
highly effective and safe for people with MS. The annual cost of
Cladribine treatment in MS would be under £1,000 compared to licensed
drugs of lesser efficacy that cost 20x or more. I would be very happy
to expand on this example further if you are interested and have the
time; just call me on my mobile xxx.
For now, I do hope you are able to support the Bill. Please let me know if there is anything further I can do to promote it.
With best wishes
Sincerely Yours,
Klaus Schmierer
I'll keep watching out for comments by my MP, over and above his auto-reply...
Thursday 22 October 2015
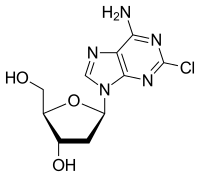
Cladribine
Cladribine (trade names Leustatin, Litak and Movectro) is a drug used to treat hairy cell leukemia (HCL, leukemic reticuloendotheliosis) and multiple sclerosis. Its chemical name is 2-chlorodeoxyadenosine (2CDA).
5-(6-Amino-2-chloro-purin-9-yl)-2-(hydroxymethyl)oxolan-3-ol
As a purine analog, it is a synthetic anti-cancer agent that also suppresses the immune system. Chemically, it mimics the nucleosideadenosine and thus inhibits the enzyme adenosine deaminase, which interferes with the cell's ability to process DNA. It is easily destroyed by normal cells except for blood cells, with the result that it produces relatively few side effects and results in very little non-target cell loss.
Cladribine was designed by Dennis A. Carson as an anti-lymphocyte compound, while he was at The Scripps Research Institute in La Jolla, California. It was first synthesized at Brigham Young University.The pharmacology and clinical applications were researched by scientists at Johnson and Johnson, which filed the New Drug Application and launched the drug in 1993.
Cladribine was designed based on information about an immune deficiency disease called adenosine deaminase deficiency. Carson described it as "a targeted agent directed against lymphocytes at a time when there was no such thing as targeted agents".
In 2008, Ernest Beutler of The Scripps Research Institute won the Wallace H. Coulter Award for Lifetime Achievement in Haematology from the Coulter Foundation and theAmerican Society of Hematology in part because of the clinical trials he ran, which established cladribine as the most effective treatment for hairy cell leukemia (HCL).
5-(6-Amino-2-chloro-purin-9-yl)-2-(hydroxymethyl)oxolan-3-ol
As a purine analog, it is a synthetic anti-cancer agent that also suppresses the immune system. Chemically, it mimics the nucleosideadenosine and thus inhibits the enzyme adenosine deaminase, which interferes with the cell's ability to process DNA. It is easily destroyed by normal cells except for blood cells, with the result that it produces relatively few side effects and results in very little non-target cell loss.
Cladribine was designed by Dennis A. Carson as an anti-lymphocyte compound, while he was at The Scripps Research Institute in La Jolla, California. It was first synthesized at Brigham Young University.The pharmacology and clinical applications were researched by scientists at Johnson and Johnson, which filed the New Drug Application and launched the drug in 1993.
Cladribine was designed based on information about an immune deficiency disease called adenosine deaminase deficiency. Carson described it as "a targeted agent directed against lymphocytes at a time when there was no such thing as targeted agents".
In 2008, Ernest Beutler of The Scripps Research Institute won the Wallace H. Coulter Award for Lifetime Achievement in Haematology from the Coulter Foundation and theAmerican Society of Hematology in part because of the clinical trials he ran, which established cladribine as the most effective treatment for hairy cell leukemia (HCL).
Thursday 1 October 2015
Unrelated Blogger Comments
Contact
NHS
- Address: Dr Klaus Schmierer, Neuroscience Clinical Academic Group, Barts Health NHS Trust, The Royal London Hospital, Whitechapel, London E1 1BB, klaus.schmierer@bartshealth.nhs.uk
- Patient pathway coordinator: Patricia Willams-Falokun, Neuroscience Clinical Academic Group, Barts Health NHS Trust, The Royal London Hospital, Whitechapel, London E1 1BB, patricia.williams-falokun@bartshealth.nhs.uk
- Neurology Infusion and Planned Investigation Unit, The Royal London Hospital, Ward 11D, 11th Floor, Central Tower, Whitechapel, London, E1 1BB, Switchboard: 020 3594 0000, Direct Dial Ward Number: 0203 5940637/0638, Ward Manager: Maria Espasandin maria.espasandin@bartshealth.nhs.uk
- freya.edwards@bartshealth.nhs.uk
- The Royal London Hospital Map: click here for map
ACADEMIC
- Address: Dr Klaus Schmierer, Blizard Institute, Queen Mary University of London, 2 Newark Street, Whitechapel, London E1 2AT. k.schmierer@qmul.ac.uk
- Laboratory Manager: David Holden,for laboratory related issues and queries in relation to grant budgets: Tel: +44 20 7882 2327, Email: d.w.holden@qmul.ac.uk
- Neuroscience & Trauma Centre Administrator: Jyoti Salhan, for issues related to Blizard Institute and the Neuroscience & Trauma Centre, Tel. +44 20 7882 8605, Fax. +44 20 77882 2180 Email: j.salhan@qmul.ac.uk
- Blizard Institute Map: click here for map
Rules of Conduct
RULES OF ENGAGEMENT
What are the do's and importantly don'ts of posting a comment.
All comments being moderated. This means they are slow to arrive and depending on content may not arrive at all.
Some we may remove even after posting, because we have not thought the issue through enough. If we have time we may redact them.
We are not here to be punchbags and we may bite back, because we are not wet blankets.
The views present on this Blog are those of the authors and have nothing to do with QMUL
We do not do the blog by Committee....We are human and we make Mistakes
We may have moments of bad judgement and we apologise for this
We do not get paid by Pharma for doing this Blog
(However. see our conflicts and judge accordingly....)
Pharma do not control the content...this is based on current research news. We do not control this.
All contributors do so Voluntarily.
We are not all Seasoned Campaigners and being rude to People
New to the Blog, May mean they never return.
We do not monitorize the Blog.
We do not make money from the Blog
- Do Not Advertise
- Do Not Include Secret Links
- Do Not Include Links to Commercial sites
- Do Not have links in your Webname
Such Posts will be deleted if they are not already caught by the Spam .
Explain what the link means, as we have to check them
Amazing information....you are so ace etc, etc as this is normally followed by "read myblog at website" or "come buy my product" and as we can not see the whole post until it is launched and posted, if a post starts with too much praise it may not see the light as these posts are spammed
We accept that sometimes we may be fooled into giving plugs
Importantly
- Do not libel
Be careful of accusations that you make.
We have fought hard to keep the option of "anonymous comments".
However, if push comes to shove even Google will give you up, as most emails/posts are traceable and it can be an criminal offence to troll.
- No Trolls Please, you make the blog an unpleasant experience
You may be very disgruntled with neuro X or person Y or place Z that but please do not identify anybody or place related to any dubious thought, activity or practice. They may have no right of reply and you could be committing libel.
- Use Dr X, Dr Y. etc. You are making a comment and not a vendetta.
- You can make gushy comments that are complementary. Ego enlargement is OK, however destructive comments are not welcome.
We realise that some people are seen as heros others less so we will try not to burst too many bubbles.
- Be constructive, even when you are making a negative comment. (This does not mean they have to be bland, we all enjoy a good debate)
- Don't shoot the messenger
- This is not a soap box (OK it is our soap box)
- If you have a long post maybe best to write and cut and paste as comments can be word limited
- This is a not a private consultation, keep your comments general. We can not do consultatations and without your notes and history it is difficult to give advice anyway.
- Please be patient. We try and answer comments but some will slip through the net. Some of the bloggers launching the comments are not qualified to answer them and answers may be slow.
- If you don't understand, ask the question! Don't be afraid of the "stupid question" because if you don't understand there will be many other readers that do not understand also. Doing this as "anonymous" should hide your embarrassment if there is an easy answer. If there is an easy answer this may be forthcoming from an MSer.
Disclaimer
Disclaimer
General Disclaimer: Please note that the opinions expressed here are those of the individual bloggers and do not necessarily reflect the positions of the Barts and The London School of Medicine and Dentistry or Barts Health NHS Trust.
Survey Disclaimer: No personal identifiers will be collected as part of these surveys. By completing these surveys you are consenting to the data you provide being analysed by DrK and his collaborators. Results of these surveys will be presented on this blog and may be submitted for publication.
Conflicts
Conflicts
As a principle we will attempt to declare all relevant conflicts of interest in relation to our postings.
However, we have many conflicts of interest and therefore read this information with this in,and an open,mind.
As a principle we will attempt to declare all relevant conflicts of interest in relation to our postings.
However, we have many conflicts of interest and therefore read this information with this in,and an open,mind.
Klaus Schmierer is a principal investigator of trials sponsored by Novartis, Roche and Teva. He is also involved in trials sponsored byBiogen, Genzyme, BIAL, has revieced speaking honoraria from, and/or served in an advisory role for, Biogen, Novartis, Teva, Merck Inc., and travel support from Genzyme to attend AAN 2014. Klaus and his group have received research grant support from Novartis, the WellcomeTrust, the National MS Society (US), the MS Society of Great Britain & Northern Ireland, the Royal College of Radiologists, and Barts Charity.
We have received grant support from companies and Aims2cure; The National Multiple Sclerosis Society, The Multiple Sclerosis Society, The Wellcome Trust; The Medical Research Council; Innovate UK; Fastforward and others, for which we are very grateful.
Updated, October 2015
Updated, October 2015
Blog Authours
DrK (Klaus Schmierer PhD FRCP)
Multiple sclerosis has been DrK's clinical and research focus from the beginning of his training in neurology at the Charité Hospital (Humboldt University), which followed undergraduate studies in Berlin and Jerusalem. In 2001 he moved to London to pursue a career in academic neurology, initially as a Research Fellow, and from 2005 as a Wellcome Intermediate Clinical Fellow at the UCL Institute of Neurology, and a Consultant Neurologist (Hon) at The National Hospital for Neurology & Neurosurgery, Queen Square. Here, he investigated the histo-pathological correlates of quantitative MRI using standard and high-field MR systems to improve disease monitoring in people with MS. Following appointment in 2009 at Queen Mary, where he is a Reader in Clinical Neurology, his clinical-academic work now includes (i) quantifying the pathological substrate of disease deterioration in pwMS using MRI and quantitative histology; (ii) further developing the BartsMS Database (the MS Trust's QuDos runner up 2015!); (iii) in vivo MRI studies to improve the early diagnosis of MS, and (iv) investigator-led and commercial clinical trials. DrK considers Cladribine the best currently available 'allround drug' for people with MS.
MouseDoctor. Has an ology.
The MouseDoctor spent his academic career based at different places of the University of London . He was awarded a BSc (Hons) in Zoology from Bedford College in 1983 and was awarded a PhD from London University in Immunology/Pathology in 1987 for work at the Institute of Basic medical Science on immunological tolerance induction in delayed hypersensitivity of the skin. He then spent six years as the Angela Limerick lecturer, for multiple sclerosis research at the Hunterian Institute, The Royal College of Surgeons of England working on delayed hypersensitivity in the brain, where he developed an active research interest in multiple sclerosis. He took a 5 year Principal Fellowship to the Institute of Ophthalmology , University College London in 1994 and became the first Senior Fellow of the Multiple Sclerosis Society and moved to the Institute of Neurology , University College London in 1999. He became a senior lecturer in 2003 and got a personal chair in 2004 as Professor of Neuroimmunology. He moved to Queen Mary in the autumn of 2006.
CannedPictures
Multiple sclerosis has been DrK's clinical and research focus from the beginning of his training in neurology at the Charité Hospital (Humboldt University), which followed undergraduate studies in Berlin and Jerusalem. In 2001 he moved to London to pursue a career in academic neurology, initially as a Research Fellow, and from 2005 as a Wellcome Intermediate Clinical Fellow at the UCL Institute of Neurology, and a Consultant Neurologist (Hon) at The National Hospital for Neurology & Neurosurgery, Queen Square. Here, he investigated the histo-pathological correlates of quantitative MRI using standard and high-field MR systems to improve disease monitoring in people with MS. Following appointment in 2009 at Queen Mary, where he is a Reader in Clinical Neurology, his clinical-academic work now includes (i) quantifying the pathological substrate of disease deterioration in pwMS using MRI and quantitative histology; (ii) further developing the BartsMS Database (the MS Trust's QuDos runner up 2015!); (iii) in vivo MRI studies to improve the early diagnosis of MS, and (iv) investigator-led and commercial clinical trials. DrK considers Cladribine the best currently available 'allround drug' for people with MS.
MouseDoctor. Has an ology.
The MouseDoctor spent his academic career based at different places of the University of London . He was awarded a BSc (Hons) in Zoology from Bedford College in 1983 and was awarded a PhD from London University in Immunology/Pathology in 1987 for work at the Institute of Basic medical Science on immunological tolerance induction in delayed hypersensitivity of the skin. He then spent six years as the Angela Limerick lecturer, for multiple sclerosis research at the Hunterian Institute, The Royal College of Surgeons of England working on delayed hypersensitivity in the brain, where he developed an active research interest in multiple sclerosis. He took a 5 year Principal Fellowship to the Institute of Ophthalmology , University College London in 1994 and became the first Senior Fellow of the Multiple Sclerosis Society and moved to the Institute of Neurology , University College London in 1999. He became a senior lecturer in 2003 and got a personal chair in 2004 as Professor of Neuroimmunology. He moved to Queen Mary in the autumn of 2006.
CannedPictures
Canned Pictures is a journalist with experience as a script writer for an Ex Prime Minster and worked on Newsnight for the BBC. They have MS.
BartsMSBlog a cyborg collective
BartsMSBlog a cyborg collective
This is a lab portal for anyone (shy members) in the group to use
About this Blog
Cladribine is an anti-Cancer drug that is used to treat hairy Cell Leukaemia.
This is available as a generic drug that is injected either via the subcutaneous route under the skin or as an intravenous infusion.
In 2010 an oral Produg of Cladribine called Movectro was Shown to be Effective in the Control of Multiple Sclerosis.
Giovannoni G, Comi G, Cook S, Rammohan K, Rieckmann P, Soelberg Sørensen P, Vermersch P, Chang P, Hamlett A, Musch B, Greenberg SJ; CLARITY Study Group.
A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis.N Engl J Med. 2010 Feb 4;362(5):416-26.
BACKGROUND:Cladribine provides immunomodulation through selective targeting of lymphocyte subtypes. We report the results of a 96-week phase 3 trial of a short-course oral tablet therapy in patients with relapsing-remitting multiple sclerosis.
METHODS:We randomly assigned 1326 patients in an approximate 1:1:1 ratio to receive one of two cumulative doses of cladribine tablets (either 3.5 mg or 5.25 mg per kilogram of body weight) or matching placebo, given in two or four short courses for the first 48 weeks, then in two short courses starting at week 48 and week 52 (for a total of 8 to 20 days per year). The primary end point was the rate of relapse at 96 weeks.
RESULTS:Among patients who received cladribine tablets (either 3.5 mg or 5.25 mg per kilogram), there was a significantly lower annualized rate of relapse than in the placebo group (0.14 and 0.15, respectively, vs. 0.33; P<0.001 for both comparisons), a higher relapse-free rate (79.7% and 78.9%, respectively, vs. 60.9%; P<0.001 for both comparisons), a lower risk of 3-month sustained progression of disability (hazard ratio for the 3.5-mg group, 0.67; 95% confidence interval [CI], 0.48 to 0.93; P=0.02; and hazard ratio for the 5.25-mg group, 0.69; 95% CI, 0.49 to 0.96; P=0.03), and significant reductions in the brain lesion count on magnetic resonance imaging (MRI) (P<0.001 for all comparisons). Adverse events that were more frequent in the cladribine groups included lymphocytopenia (21.6% in the 3.5-mg group and 31.5% in the 5.25-mg group, vs. 1.8%) and herpes zoster (8 patients and 12 patieTreatment with cladribine tablets significantly reduced relapse rates, the risk of disability progression, and MRI measures of disease activity at 96 weeks. The benefits need to be weighed against the risks. (ClinicalTrials.gov number, NCT00213135.)
Giovannoni G, Cook S, Rammohan K, Rieckmann P, Sørensen PS, Vermersch P, Hamlett A, Viglietta V, Greenberg S; CLARITY study group. Sustained disease-activity-free status in patients with relapsing-remitting multiple sclerosis treated with cladribine tablets in the CLARITY study: a post-hoc and subgroup analysis. Lancet Neurol. 2011 Apr;10(4):329-37
Cook S, Vermersch P, Comi G, Giovannoni G, Rammohan K, Rieckmann P, Sørensen PS, Hamlett A, Miret M, Weiner J, Viglietta V, Musch B, Greenberg SJ; CLARITY Study Group.Safety and tolerability of cladribine tablets in multiple sclerosis: the CLARITY (CLAdRIbine Tablets treating multiple sclerosis orallY) study.Mult Scler. 2011 May;17(5):578-93.
Comi G, Cook SD, Giovannoni G, Rammohan K, Rieckmann P, Sørensen PS, Vermersch P, Hamlett AC, Viglietta V, Greenberg SJ.MRI outcomes with cladribine tablets for multiple sclerosis in the CLARITY study.J Neurol. 2013 Apr;260(4):1136-46
However following the request of the the Regulators for more data the Company manufacturing the drug stopped production and ceased interest
This is available as a generic drug that is injected either via the subcutaneous route under the skin or as an intravenous infusion.
In 2010 an oral Produg of Cladribine called Movectro was Shown to be Effective in the Control of Multiple Sclerosis.
Giovannoni G, Comi G, Cook S, Rammohan K, Rieckmann P, Soelberg Sørensen P, Vermersch P, Chang P, Hamlett A, Musch B, Greenberg SJ; CLARITY Study Group.
A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis.N Engl J Med. 2010 Feb 4;362(5):416-26.
BACKGROUND:Cladribine provides immunomodulation through selective targeting of lymphocyte subtypes. We report the results of a 96-week phase 3 trial of a short-course oral tablet therapy in patients with relapsing-remitting multiple sclerosis.
METHODS:We randomly assigned 1326 patients in an approximate 1:1:1 ratio to receive one of two cumulative doses of cladribine tablets (either 3.5 mg or 5.25 mg per kilogram of body weight) or matching placebo, given in two or four short courses for the first 48 weeks, then in two short courses starting at week 48 and week 52 (for a total of 8 to 20 days per year). The primary end point was the rate of relapse at 96 weeks.
RESULTS:Among patients who received cladribine tablets (either 3.5 mg or 5.25 mg per kilogram), there was a significantly lower annualized rate of relapse than in the placebo group (0.14 and 0.15, respectively, vs. 0.33; P<0.001 for both comparisons), a higher relapse-free rate (79.7% and 78.9%, respectively, vs. 60.9%; P<0.001 for both comparisons), a lower risk of 3-month sustained progression of disability (hazard ratio for the 3.5-mg group, 0.67; 95% confidence interval [CI], 0.48 to 0.93; P=0.02; and hazard ratio for the 5.25-mg group, 0.69; 95% CI, 0.49 to 0.96; P=0.03), and significant reductions in the brain lesion count on magnetic resonance imaging (MRI) (P<0.001 for all comparisons). Adverse events that were more frequent in the cladribine groups included lymphocytopenia (21.6% in the 3.5-mg group and 31.5% in the 5.25-mg group, vs. 1.8%) and herpes zoster (8 patients and 12 patieTreatment with cladribine tablets significantly reduced relapse rates, the risk of disability progression, and MRI measures of disease activity at 96 weeks. The benefits need to be weighed against the risks. (ClinicalTrials.gov number, NCT00213135.)
Giovannoni G, Cook S, Rammohan K, Rieckmann P, Sørensen PS, Vermersch P, Hamlett A, Viglietta V, Greenberg S; CLARITY study group. Sustained disease-activity-free status in patients with relapsing-remitting multiple sclerosis treated with cladribine tablets in the CLARITY study: a post-hoc and subgroup analysis. Lancet Neurol. 2011 Apr;10(4):329-37
Cook S, Vermersch P, Comi G, Giovannoni G, Rammohan K, Rieckmann P, Sørensen PS, Hamlett A, Miret M, Weiner J, Viglietta V, Musch B, Greenberg SJ; CLARITY Study Group.Safety and tolerability of cladribine tablets in multiple sclerosis: the CLARITY (CLAdRIbine Tablets treating multiple sclerosis orallY) study.Mult Scler. 2011 May;17(5):578-93.
Comi G, Cook SD, Giovannoni G, Rammohan K, Rieckmann P, Sørensen PS, Vermersch P, Hamlett AC, Viglietta V, Greenberg SJ.MRI outcomes with cladribine tablets for multiple sclerosis in the CLARITY study.J Neurol. 2013 Apr;260(4):1136-46
However following the request of the the Regulators for more data the Company manufacturing the drug stopped production and ceased interest
What happened next. Read this Blog.
CONCLUSIONS:
Subscribe to:
Posts (Atom)